| 11. |
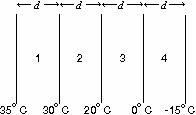
The diagram shows four slabs of different materials with
equal thickness, placed side by side. Heat flows from left to right and
the steady-state temperatures of the interfaces are given. Rank the
materials according to their thermal conductivities, smallest to
largest.
|